I have always seen the beauty of chemistry from a scientific standpoint: strange shapes, eye-catching patterns, giant explosions… But it was not until I came to Wolfram|Alpha that I began to appreciate just how sleek chemistry is from a programming perspective. Just a few lines of code are needed to create some of the most startling phenomena and give life to elegant theories. More »
Wolfram|Alpha is a great resource for learning about chemical reactions. We recently rolled out a brand new interface that allows you to easily search through our large database of reactions and explore classes of chemical reactions (such as combustion or oxidation reactions). And we also introduced new Step-by-step functionality to illustrate how to balance chemical reactions. More »
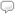 10
10
Wolfram|Alpha Pro has become well known for its ability to show not only the answer to a question, but also the Step-by-step solution to show how to find the answer. Since its first release, we have developed new features and content for Step-by-step solutions. We’ve added hints and the ability to walk through problems one step at a time, and we’ve added support to show multiple methods for solving problems whenever possible. More »
Bonding is pretty fundamental, as it determines the shape of a molecule, which in turn determines how a molecule behaves. Wolfram|Alpha now supports Lewis structures—diagrams that show both the bonded and unbonded electrons in a molecule. But as any good teacher will tell you, even more important than finding the answer is the procedure used to get that answer. Thus, Wolfram|Alpha is expanding its Step-by-step interface into the realm of chemical bonding (with even more Step-by-step functionality coming soon). More »
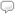 2
2
Superlatives, like hyperbole, are my favorite thing. So it is with the greatest excitement that I am devoting this blog post to superlatives and range searching, as Wolfram|Alpha has again expanded its functionality in these areas.
I once heard from an actor pretending to be a scientist that the denser an element is, the better that element is for fighting terrible monsters. I cannot speak on the accuracy of that statement, as I am not an actor pretending to be a scientist, but if you wanted to apply superlatives to chemistry, Wolfram|Alpha can do that. More »
The Nobel Prizes, named for chemist and engineer Alfred Nobel, inventor of dynamite and activist for humankind’s advancement, were handed out in Stockholm today. Wolfram|Alpha can tell us that the winners include Brian Kobilka and Robert Lefkowitz for the Nobel Prize in Chemistry.
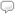 2
2
Today is National Mole Day. Unlike most of Wolfram|Alpha’s team I’m not a scientist, so if you’re curious to learn what the day is about, so am I—we can learn together! Thankfully, Wolfram|Alpha is a great tool for learning pretty much anything.
We here at Wolfram|Alpha are constantly trying to improve the user experience by fine-tuning our algorithms and making our functionality in every domain more versatile and flexible. We are pleased to announce that we have made useful upgrades to chemistry functionality in Wolfram|Alpha, especially in the domain of solution chemistry. We have new data that enables you to quickly determine whether a given set of solvents are miscible in each other or not: “Is acetone miscible in benzene?” You also could ask for the list of liquids that are miscible in a given solvent: “What solvents are miscible in acetone?” We are improving our coverage of this area, with new data being added regularly.
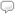 6
6
Do you need some help navigating your chemistry or precalculus classes? Or maybe you’re still trying to decide which classes to take this fall. Good news! Today, we’re releasing the Wolfram General Chemistry and Precalculus Course Assistant Apps, two more Wolfram|Alpha-powered course assistants that will help you better understand the concepts addressed in these classes.
If you’re taking chemistry, download the Wolfram General Chemistry Course Assistant App for everything from looking up simple properties like electron configurations to computing the stoichiometric amounts of solutes that are present in solutions of different concentrations. This app is handy for lab researchers, too!
The specialized keyboard allows you to enter chemicals by using formulas or by spelling out their names.
More »
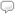 5
5
When we are growing up and learning about the world, there are moments when a topic or idea really catches our attention. Perhaps it is while reading a book or during a lecture given by a good teacher. For me, one of those moments occurred during my junior year of high school in Mr. Brooks’s chemistry class. We were learning about the structure of the atom, and Mr. Brooks did a demonstration for us. He turned off the lights in the classroom and turned on a hydrogen discharge tube. The tube glowed with a pink light. Then Mr. Brooks put a prism in front of the glowing discharge tube, and several vertical lines of light appeared on the chalk board behind the prism.
At the time, I didn’t really understand that the voltage applied across the discharge tube was exciting the electrons around the hydrogen atoms and that the lines formed as the pink light passed through the prism were characteristic wavelengths of light being emitted as the electrons around the hydrogen atoms returned to lower energy levels. But I clearly remember the intense curiosity I felt about the phenomenon I was witnessing. It is, therefore, with some nostalgia that I announce the addition of the National Institute of Standards and Technology’s (NIST) atomic spectra database to Wolfram|Alpha.
Investigation of atomic spectra contributed significantly to our understanding of atomic structure and are described by the Rydberg formula. Furthermore, atomic spectra are used by astronomers to classify and determine the composition of stars. Today, the NIST database has become the most comprehensive and reliable set of data for atomic spectra and includes information about spectral lines and atomic energy levels associated with many elements and ions. All of this data can now be found in Wolfram|Alpha, including that visible hydrogen spectrum I was so curious about in high school:
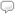 4
4
A few months back we introduced our blog readers to Wolfram|Alpha’s chemistry data, and we thought it would be fitting to have a Chemistry 101 review blog post for Homework Day. Wolfram|Alpha contains a wealth of chemistry data, and provides you with rapid and accurate computations at the simple push of a button. Wolfram|Alpha is an incredible learning tool for new chemistry students looking for ways to learn and test their knowledge of chemistry basics. Many of the topic areas found in an introductory or basic chemistry course syllabus can be explored in Wolfram|Alpha.
Need to compute how many moles are in 5 grams of iron? Query “how many moles are in 5 grams of iron?“, and Wolfram|Alpha quickly computes your input and returns a result, along with unit conversions.
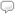 3
3
On Monday, we kicked off our series on using Wolfram|Alpha for chemistry in honor of the American Chemical Society’s Fall 2009 National Meeting & Exposition, taking place in Washington, DC, USA this week. In this post, we begin to break down chemistry topics by taking a look Wolfram|Alpha’s collection of chemical element data. If you are attending the meeting, stop by the Wolfram Research booth, #2101, for a personal introduction to Wolfram|Alpha and the technology behind it.
The periodic table and its elements can be viewed as the foundation for building your knowledge and understanding of chemistry. Wolfram|Alpha defines a chemical element as any of the more than 100 known substances that cannot be separated into simpler substances and that singly or in combination constitute all matter. Currently, there are 118 commonly recognized elements, 92 of which occur naturally, and the others synthetically. The periodic table is organized in 18 columns (called groups) and 7 rows (called periods). Elements are arranged in the table based on their atomic weight.
In Wolfram|Alpha you can retrieve data for a chemical element in a number of ways, such as by name, symbol, atomic number, or a specific class, such as radioactive elements. In this example we query “hydrogen” and quickly learn from the basic elemental properties pod that it has an atomic number of one, which places it in the first position on the periodic table. We also learn its symbol, atomic weight, thermodynamic properties, material properties, electromagnetic properties, reactivity, atomic properties, abundances, nuclear properties, and identifiers. Click the image to explore more properties of hydrogen.
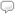 12
12
This week the American Chemical Society (ACS) is holding its Fall 2009 National Meeting & Exposition in Washington, DC, USA. In honor of professional chemists, educators, and students, we’re celebrating chemistry this week. If you are attending the meeting and would like a personal introduction to Wolfram|Alpha or the technology behind it, drop by the Wolfram Research booth, #2101.
Wolfram|Alpha contains a wealth of chemistry data, and provides you rapid computations that ensure accuracy and save time. Wolfram|Alpha is also an incredible learning tool, especially for new chemistry students looking for ways to learn, understand, compare, and test their knowledge of chemistry basics. Many of the topic areas found on an introductory or advanced course syllabus can be explored in Wolfram|Alpha.
Need to compute how many moles are in 5 grams of iron? Query “how many moles are in 5 grams of iron?”, and Wolfram|Alpha quickly computes your input and returns a result, along with unit conversions.
Need some quick facts about carbon? With a quick query, Wolfram|Alpha returns its periodic table location, thermodynamic and material properties, and much more.

Here is an example of how you can save time by converting properties in Wolfram|Alpha:

With Wolfram|Alpha you can explore additional areas of basic chemistry such as computing a unit conversion, referencing chemical elements, ions, chemical compounds, thermodynamics, quantities of chemicals, and chemical solutions.
In Wednesday’s blog post we will break down chemistry topic areas and explore how Wolfram|Alpha can help you work through specific exercises, such as identifying and comparing classes of chemical elements, calculating thermodynamics, preparing solutions, converting units, and stoichiometry. Are you a professional who is using Wolfram|Alpha in your research today? Are you an instructor who has incorporated Wolfram|Alpha into your classroom, or a student who is using it to prepare for your chemistry courses? Share your experiences with other chemistry enthusiasts having this conversation on the Wolfram|Alpha Community site.








